Korea Medical Device Regulations. Medical Technology Magazine The medical device industrys most comprehensive news and information delivered every month.

Top 5 Environmental Stats Industrial Water Pollution Infographic Intelex Water Waterquality Waterpollution Pol Water Pollution Pollution Environment
As a general rule as the associated risk of the device.

. A1-2 Block A Jalan Haruan 59 Pusat Komersial Oakland II 70300 Seremban Negeri Sembilan Malaysia Seremban Negeri Sembilan 70300 Malaysia. Guidance Document under Medical Device Act 2012 Act 737 These Guidance Document was prepared by the Medical Device Authority MDA to help the industry and healthcare professionals in their quest to comply with the Medical Device Act Act 737 and the regulations under it. However the few products for which an SDS is.
An Act to provide for the control of factories with respect to matters relating to the safety health and welfare of person therein the registration and inspection of machinery and for matters connected therewith. Medical Device Network Daily Update The top stories of the day delivered to you every weekday. 1 February 1970 PU.
Almost all Fresenius Kabi medical devices are non-hazardous and therefore do not require SDS sheets. This Guidance Document shall be read in conjunction with the current laws. B 51970 PART I PRELIMINARY 1.
Medical devices in Brazil are regulated by the National Health Surveillance Agency ANVISA. The cost of diagnostic testing and medications is particularly expensive in the United States. This includes labor costs for nurses aides surgeons pharmacists physical therapists and more.
Brazils base regulations and medical device classification schemes are similar to those found in the European MDD 9342EEC. After July 1 2021 risk classification system and new. Call for comments.
Medical Device Listing in Medical Device National Registry MDNR 4 working days Class A. Office 32 19-21 Crawford Street London W1H 1PJ UK Responsible Person United Kingdom. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assurance before regulating governments allow marketing of the device in their country.
Medical Device Network Weekly Roundup A weekly roundup of the latest news and analysis sent every Friday. The MFDS periodically releases relevant notifications that cover more detailed technical requirements. Current Korea medical device regulations are covered under the Medical Device Act MDA that went into effect in 2004.
However registering a device or IVD in Mexico can be challenging for non-Spanish speakers. Foreign manufacturer must appoint a company residing within the KSA to act on their behalf for specified tasks including dealing with the SFDA. Our Medical Centre has been specifically designed to supply the most comprehensive combination of specialized techniques all in one location within a relaxing environment complete with welcoming and caring staff.
Malaysia-Singapore total trade to hit RM481bil in 2022 September 10 2022 1146 AM. Sign any letter word name signature numeral device brand heading label ticket shape of goods or their packaging color sound scent hologram positioning. Guidance Document under Medical Device Act 2012 Act 737 These Guidance Document was prepared by the Medical Device Authority MDA to help the industry and healthcare professionals in their quest to comply with the Medical Device Act Act 737 and the regulations under it.
This Guidance Document shall be read in conjunction with the current laws. CMC Medical Devices LTD. COFEPRIS the division of the Mexican Ministry of Health responsible for medical device and IVD oversight provides limited.
We can combine Hyperbaric Oxygen Therapy with Extracorporeal blood oxygenation and ozonation EBOO for Anti-Aging. 10 working days for Class B C D. Various amendments and modifications have been made to the MDA since its initial release.
High cost of malpractice insurancethe insurance that protects medical professionals against. Trademark means any sign capable of being represented graphically which is capable of distinguishing goods or services of one undertaking from those of other undertakings. Brazil is the largest medical device market in Latin America with an established but complex regulatory system.
A medical device is any device intended to be used for medical purposes. 1 This Act may be cited as the Factories and Machinery Act. The cost of pre- and post-procedure labor is often dramatically lower overseas.
A Safety Data Sheet SDS provides data regarding the properties of medical device products. Medical Device Regulatory Authorities Training Curriculum White Paper Submitted by admin on Wed 07272022 - 0943 If you have any comments on the draft of Medical Device Regulatory Authorities Training Curriculum White Paper please send them to the GHWP Secretariat secretariatghwpinfo with the template below on or before 17 August. Mexico is the second-largest medical device market in Latin America after Brazil and can be a profitable target for medical device and IVD manufacturers.
Malaysian Cocoa Board director-general Ramle Kasin says Malaysias annual cocoa production is only 100000 tonnes.
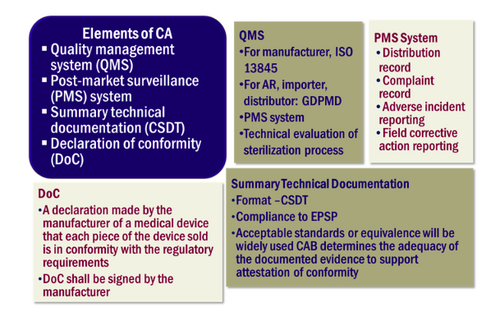
General Medical Device Medical Device Authority Mda

Patent Manual Application Flowchart Flow Chart Patent Registration Application
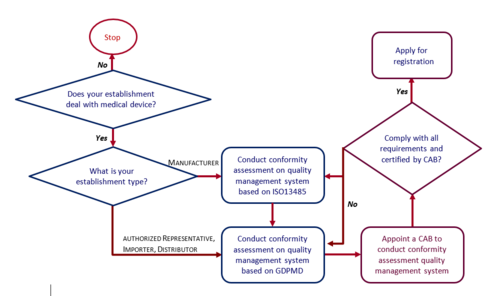
How To Apply For Establishment Licence Medical Device Authority Mda

Pin By Choi Yoke Keng On Fb Recipes Yams Recipes Spices
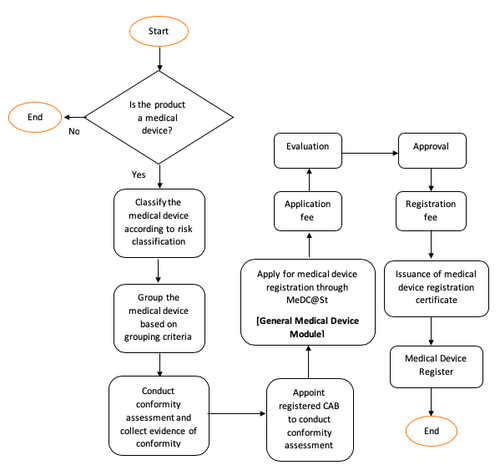
General Medical Device Medical Device Authority Mda

Pharmaboardroom Preclinical Clinical Trial Requirements Malaysia

Biosafety And Biosecurity Month Webinar Series University Programs Greetings For The Day Education Domain
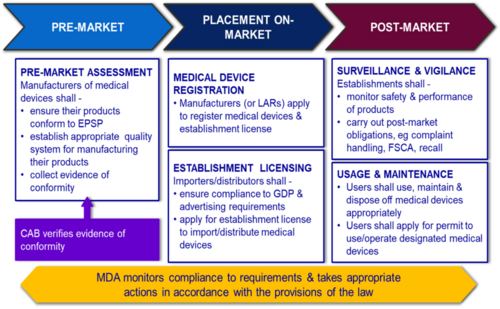
General Medical Device Medical Device Authority Mda

100glamorousjobs Jobs Career Actor Actress By Jobscentral Careerbuilder Malaysia An Actor Alternatively Act Career Builder Actors Actresses Actors
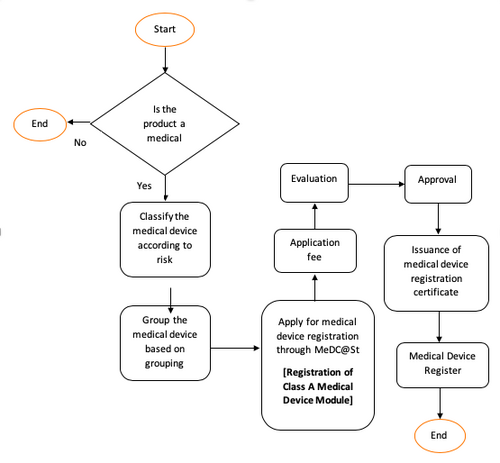
General Medical Device Medical Device Authority Mda

Editable Power Of Attorney Authorization Letter Power Of Attorney Lettering Power

Company Secretary Services Company Secretary Accounting Services Company

Medical Device Registration In Malaysia
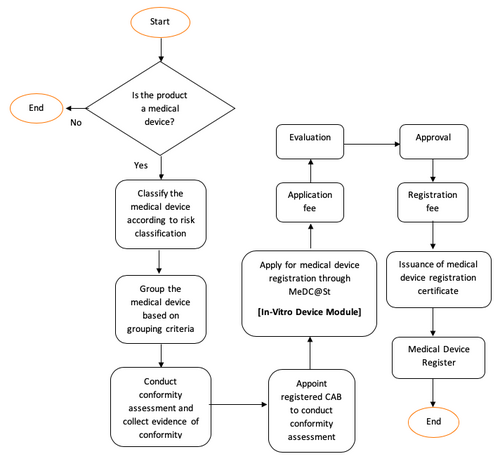
In Vitro Diaganostic Device Medical Device Authority Mda
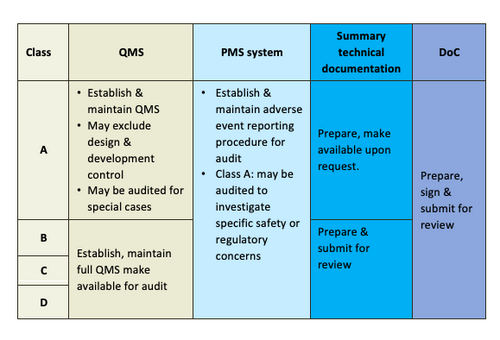
General Medical Device Medical Device Authority Mda

Mda On Complaint Handling And Problem Reporting

Infection Prevention And Control Saint Mary S Hospital Infection Prevention Hand Hygiene Prevention


